Address
10 place de la Joliette
Atrium 10.4 – Etage 6
13002 Marseille, France
Coherent Raman imaging has established itself as a powerful imaging technique in both research and clinical domains. Its ability to target specific molecular bonds enables highly specific imaging, opening up exciting possibilities in histological analysis and cellular imaging. At Lightcore Technology, we’re pushing these capabilities forward by bringing coherent anti-Stokes Raman scattering (CARS) imaging to a miniaturized, endoscopic format, tackling key technical challenges like four-wave mixing (FWM) background interference—a persistent obstacle in CARS imaging. Initial studies using femtosecond lasers confirmed the feasibility of this approach, but their short pulses, while delivering high peak power, limit spectral resolution. This hinders the discrimination of spectrally similar species within tissues.
Our research now focuses on integrating a compact, stable picosecond laser source for endoscopic CARS imaging. Partnering with Refined Laser Systems (RLS), we’ve incorporated the PICUS DUO EOM laser into our multiphoton endoscope, delivering pulses in the 1-4 ps range at 40 MHz. This pulse range provides an ideal compromise: ample peak power with enhanced spectral resolution. For this preliminary experiments, we set the wavenumber to 2850 cm⁻¹, targeting CH₂ bonds which are abundant in lipid-rich structures. Average powers were set at 50 mW for the pump (799 nm) and 90 mW for the Stokes (1034 nm), with images collected in epi-mode, ensuring a true endoscopic configuration.
In our initial trials with fixed tissue samples, the endoscope captured detailed structures across a range of tissues. Elastic fibers in the aorta revealed well-defined elastin patterns, while dry bone samples illustrated the lipid-rich matrix in sharp detail. Imaging brain tissue enabled visualization of neuronal structures, glial cells, and myelin sheaths, and imaging cardiac tissue captured organized cardiomyocytes and blood vessels. These results showcase the system’s resolution and detail, with clear differentiation across a range of tissue types.
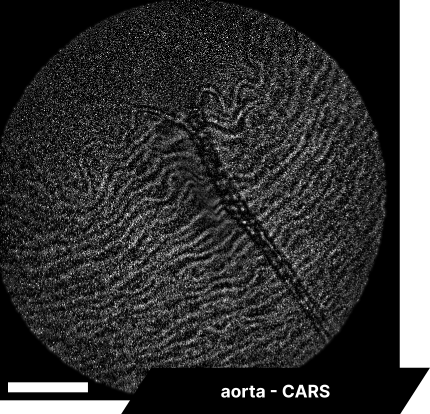
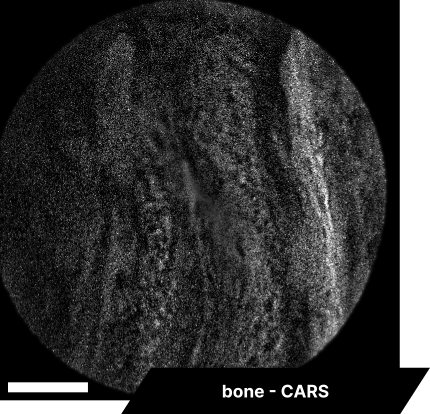
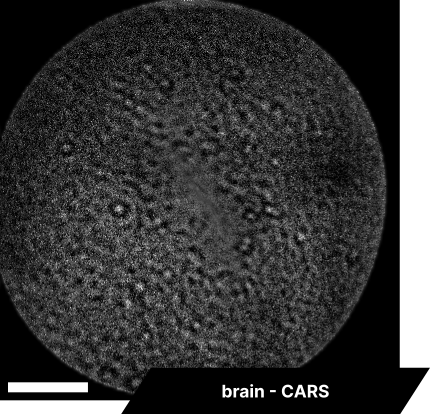
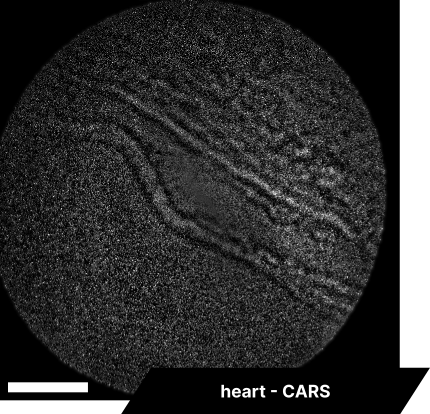
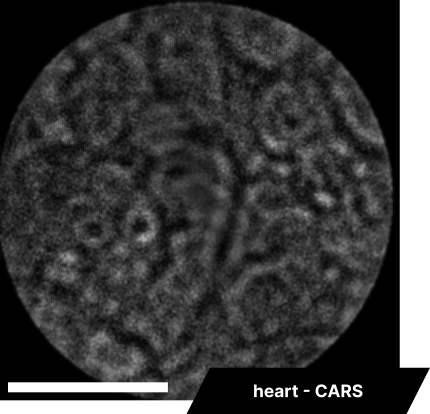
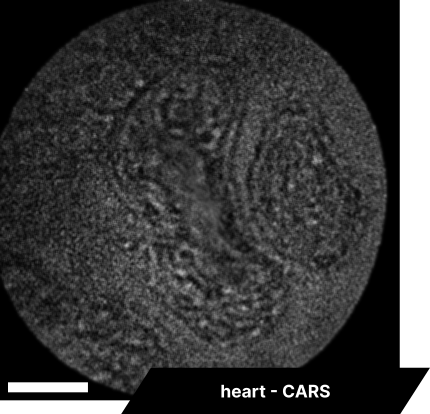
As real-time imaging is essential for practical applications, we also demonstrated the endoscope’s ability to image fixed tissues in a live setting. We observed dense connective tissue, showing elastic fibers, and noted the absence of signal from collagen due to the focus on CH₂ bonds. Additionally, lymphatic structures and vessel interconnections typical of lymphatic anatomy were visible, reflecting the device’s capability to differentiate based on molecular content.


Lastly, we conducted in-vivo imaging on human abdominal skin using a custom pen endoscope, capturing structural detail approximately 150 µm beneath the skin surface. These images reveal a promising glimpse of cellular organizations within the dermis and possible sebaceous glands. The in-vivo capability of our endoscope opens a pathway for non-invasive, real-time examination of human tissues with molecular specificity, offering substantial potential for histological analysis, dermatological applications, and beyond.
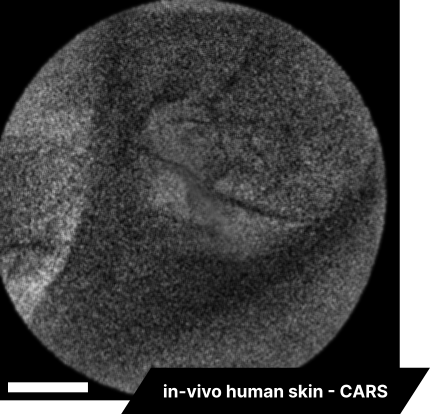
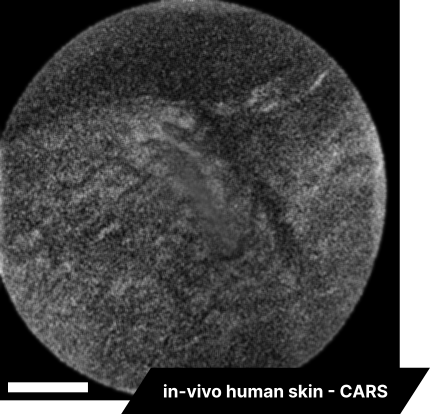
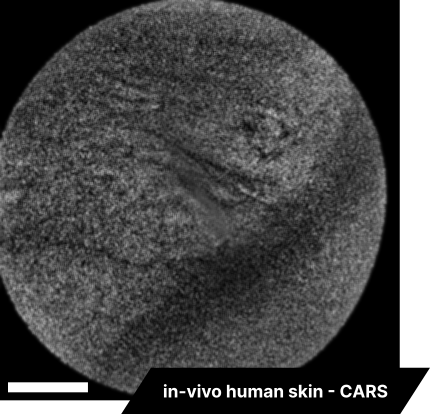
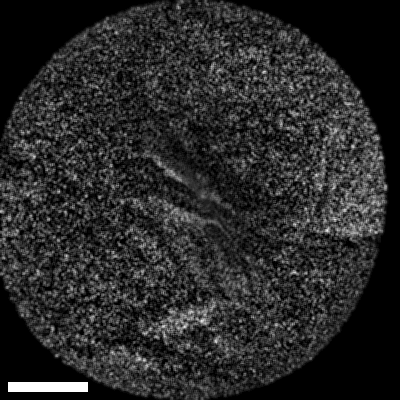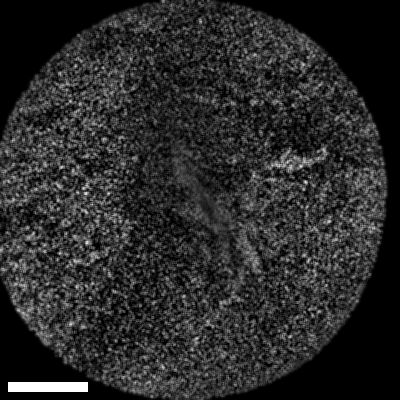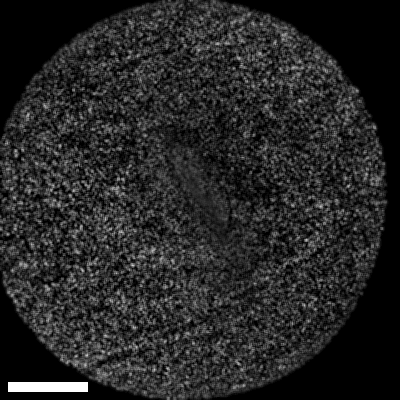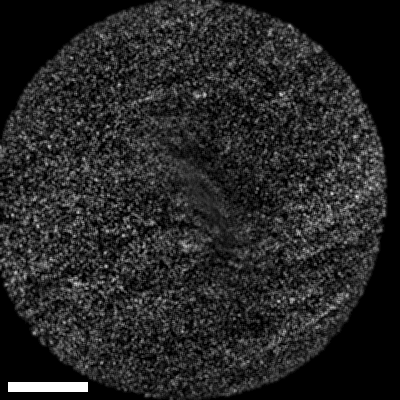
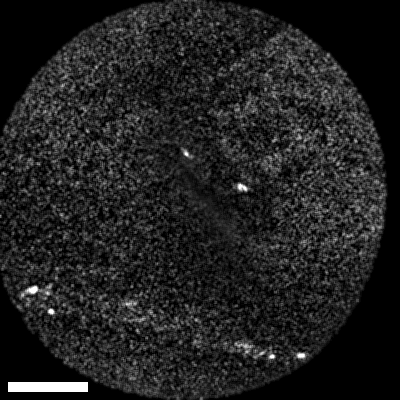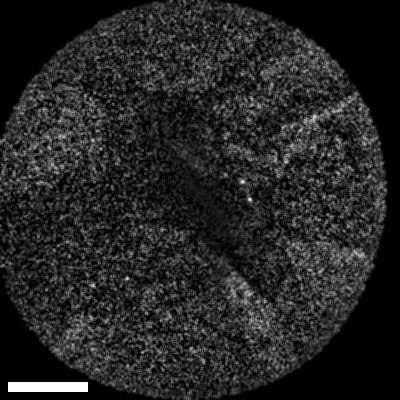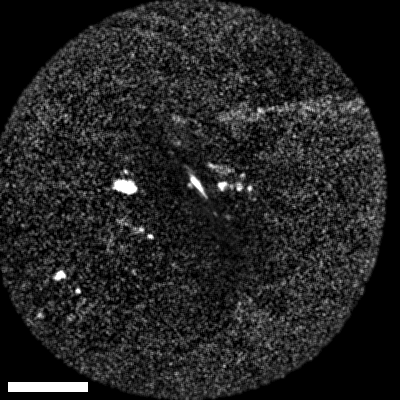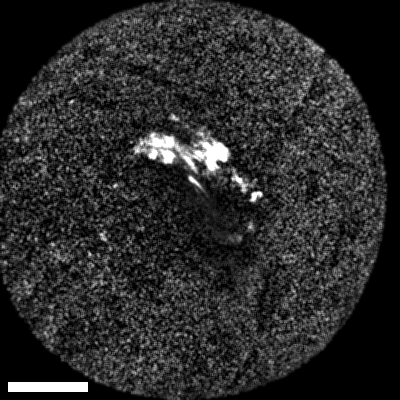
The scale bar on all above images represents 60 µm.
These early findings demonstrate our progress in achieving spectrally resolved Raman imaging for endoscopy. Our technology promises a new approach to histological imaging, with implications across both research and clinical applications. We are excited to explore the future implications of this technology as we continue to refine and expand its capabilities.


